Bihar Board 12th Chemistry Objective Questions and Answers
Bihar Board 12th Chemistry Objective Answers Chapter 5 Surface Chemistry
Question 1.
Which of the following is less than zero during adsorption ?
(a) ΔG
(b) ΔS
(c) ΔH
(d) All of these
Answer:
(d) All of these
Question 2.
Which of the following is a property of physisorption ?
(a) High specificity
(b) Irreversibility
(c) Non-specificity
(d) None of these
Answer:
(c) Non-specificity
![]()
Question 3.
Which of the following statement is not correct about physisorption ?
(a) It is a reversible process
(b) It requires less heat of adsorption
(c) It requires activation energy
(d) It takes place at low temperature
Answer:
(c) It requires activation energy
Question 4.
The incorrect statement about physical adsorption is
(a) it lacks specificity
(b) it is generally reversible
(c) porous surfaces are good adsorbent
(d) heat of adsorption is quite high
Answer:
(d) heat of adsorption is quite high
Question 5.
Which is correct in case of van der Waals adsorptions ?
(a) High temperature, low pressure
(b) High temperature, high pressure
(c) Low temperature, low pressure
(d) Low temperature, high pressure
Answer:
(d) Low temperature, high pressure
Question 6.
Which, of the following gases is least absorbed on charcoal ?
(a)HCl
(b) NH3
(c) O2
(d) CO2
Answer:
(c) O2
![]()
Question 7.
Which of the following is not characteristic of chemisorption ?
(a) Adsorption is specific
(b) Heat of adsorption is of the order of 200 kJ mol-1
(c) Adsorption is irreversible
(d) Adsorption may be multimolecular layers
Answer:
(d) Adsorption may be multimolecular layers
Question 8.
Which of the following is not correct regarding the adsorption of a gas on the surface of solid ?
(a) On increasing pressure, adsorption keeps on increasing
(b) Enthalpy and entropy changes are negative
(c) Chemisorption is more specific than physisorption
(d) It is a reversible reaction
Answer:
(a) On increasing pressure, adsorption keeps on increasing
Question 9.
Which of the plots is adsorption isobar for chemisorption?
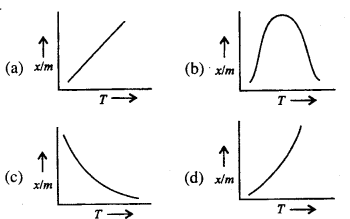
Answer:
(b)
Question 10.
Which of the following graphs would yield a stright line ?
(a) x/m vs p
(b) log x/m vs p
(c) x/m vs log p
(d) log x/m vs log p
Answer:
(d) log x/m vs log p
Question 11.
At low pressure, the fraction of the surface covered follows
(a) zero-order kinetics
(b) first order kinetics
(c) second order kinetics
(d) fractional order kinetics
Answer:
(b) first order kinetics
![]()
Question 12.
Which of the following gases present in a polluted area will be adsorbed most easily on the charcoal gas mask ?
(a) H2
(b) O3
(c) N2
(d) SO2
Answer:
(d) SO2
Question 13.
Which of the following is application of adsorption in chemical analysis
(a) Adsorption indicators
(b) Thin layer chromatography
(c) Qualitative analysis
(d) All of these
Answer:
(d) All of these
Question 14.
What is the role of adsorption in froth floatation process used especially for concentration of sulphide ores ?
(a) Shape selective catalysts
(b) Adsorption of pine oil on sulphide ore particles
(c) Adsorption of pine oil on impurities
(d) Production of heat in the process of exothermic reaction
Answer:
(b) Adsorption of pine oil on sulphide ore particles
Question 15.
Which of the following can adsorb larger volume of hydrogen gas ?
(a) Finely divided platinum
(b) Colloidal solution of palladium
(c) Small pieces of palladium
(d) A single metal surface of platinum
Answer:
(b) Colloidal solution of palladium
Question 16.
Which kind of catalysis can be explained on the basis of adsorption theory ?
(a) Homogeneous catalysis
(b) Heterogeneous catalysis
(c) Negative catalysis
(d) Auto catalysis
Answer:
(b) Heterogeneous catalysis
![]()
Question 17.
The oxide of nitrogen which acts as a catalyst in lead chamber proces is …………
(a) NO
(b) NO2
(c) N2O4
(d) N2O5
Answer:
(a) NO
Question 18.
Shape-selective catalysis is a reaction catalysed by …………….
(a) zeolites
(b) enzymes
(c) platinum
(d) Ziegler-Natta catalyst
Answer:
(a) zeolites
Question 19.
The activity of an enzyme becomes ineffective ……….
(a) at low temperature
(b) at atmospheric pressure
(c) at high temperature
(d) in aqueous medium
Answer:
(c) at high temperature
Question 20.
The size of colloidal particles ranges between ………….
(a) 10-7 – 10-9cm
(b) 10-9 -101 cm
(c) 10-5 – 10-7cm
(d) 10-2 – 10-3cm
Answer:
(c) 10-5 – 10-7cm
![]()
Question 21.
Which of the following will not form a colloidal system ?
(a) Solid-gas
(b) Liquid-gas
(c) Gas-gas
(d) Gas-liquid
Answer:
(c) Gas-gas
Question 22.
Fog is an example of colloidal system of ………….
(a) liquid in gas
(b) gas in liquid
(c) solid in gas
(d) gas in solid
Answer:
(a) liquid in gas
Question 23.
A colloidal system in which liquid is dispersed phase and solid is dispersion medium is classified as ………….
(a) gel
(b) sol
(c) emulsion
(d) aerosol
Answer:
(a) gel
Question 24.
Which of the following example is correctly matched ?
(a) Butter – gel
(b) Smoke – emulsion
(c) Paint – foam
(d) Milk – aerosol
Answer:
(a) Butter – gel
![]()
Question 25.
Which of the following factors contribute towards higher stability of lyophilic colloid ?
(a) Charge on their particles
(b) Attractive forces between particles
(c) Small size of their particles
(d) High solvation due to a layer of dispersion medium
Answer:
(d) High solvation due to a layer of dispersion medium
Question 26.
A lyophobic colloid cannot be formed by ………….
(a) mixing dispersed phase and dispersion medium
(b) chemical reactions like hydrolysis
(c) exchange of solvent
(d) geptisation
Answer:
(a) mixing dispersed phase and dispersion medium
Question 27.
Which of the following is not correctly matched ?
(a) Gelatin – Lyophilic colloid
(b) Gold sol – Lyophilic colloid
(c) Arsenious sulphide – Lyophobic colloid
(d) Ferric hydroxide – Lyophobic colloid
Answer:
(b) Gold sol – Lyophilic colloid
Question 28.
The substances which behave as colloidal solutions at higher concentration are called ………….
(a) associated colloids
(b) multimolecular colloids
(c) macromolecular colloids
(d) protective colloids
Answer:
(a) associated colloids
![]()
Question 29.
The formation of micelles takes place only above …………..
(a) critical temperature
(b) Kraft temperature
(c) inversion temperature
(d) absolute temperature
Answer:
(b) Kraft temperature
Question 30.
The critical micelle concentration (CMC) is defined as…………….
(a) the concentration at which micellization starts
(b) the concentration at which micelle starts behaving like an electrolyte
(c) the concentration at which dispersed phase is separated from dispersion medium
(d) the concentration at which a colloid is converted to suspension
Answer:
(a) the concentration at which micellization starts
Question 31.
At CMC (critical micelle concentration) the surface molecules
(a) dissociate
(b) associate
(c) become bigger in size due to adsorption
(d) become smaller in size due to decomposition
Answer:
(b) associate
Question 32.
Soap mixed with water below critical micelle concentration behaves as
(a) associated colloid
(b) macromolecular colloid
(c) normal electrolytic solution
(d) multimolecular colloid
Answer:
(c) normal electrolytic solution
Question 33.
White of an egg whipped with water acts as …………………
(a) macromolecular colloid
(b) associated colloid
(c) molecular colloid
(d) normal electrolytic solution
Answer:
(a) macromolecular colloid
![]()
Question 34.
Which of the processes is being shown in the figure ?
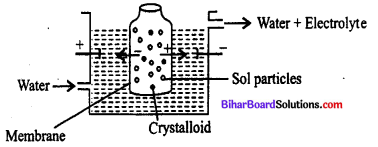
(a) Electrodialysis
(b) Dialysis
(c) Electroosmosis
(d) Eelctrophoresis
Answer:
(a) Electrodialysis
Question 35.
Which of the following is not a method of removing impurities from a colloidal sol ?
(a) Electrodialysis
(b) Ultrafiltration
(c) Ultra centrifugation
(d) Distillation
Answer:
(d) Distillation
Question 36.
Tyndall effect is not observed in ………………
(a) smoke
(b) emulsions
(c) sugar solution
(d) gold sol
Answer:
(c) sugar solution
Question 37.
Which of the following systems will show Tyndall effect ?
(a) Aqueous solution of sodium chloride
(b) Aqueous solution of aluminium hydroxide
(c) Aqueous solution of glucose
(d) Aqueous solution of urea
Answer:
(b) Aqueous solution of aluminium hydroxide
Question 38.
Which of the following is not an explanation for the origin of charge on the colloidal particles ?
(a) Due to frictional electrification
(b) Due to dissociation of surface molecules
(c) Due to electrophoresis
(d) Due to selective adsorption of ions
Answer:
(c) Due to electrophoresis
![]()
Question 39.
Movement of dispersion medium under the influence of electric field is known as ……………
(a) electrodialysis
(b) electrophoresis
(c) electroosmosis
(d) cataphoresis
Answer:
(c) electroosmosis
Question 40.
Why is alum added to water containing suspended impurities ?
(a) To make a colloidal solution
(b) To coagulate the suspended impurities
(c) To remove impurities of calcium and magnesium
(d) To protect the colloidal solution from getting precipitated
Answer:
(b) To coagulate the suspended impurities
Question 41.
Which of the following acts as the best coagulating agent for ferric hydroxide sol ?
(a) Potassium ferrocyanide
(b) Potassium chloride
(c) Potassium oxalate
(d) Aluminium chloride
Answer:
(a) Potassium ferrocyanide
Question 42.
Why is gelatin mixed with gold sol ?
(a) Gold sol is lyophobic sol, gelatin acts as stabilising agent
(b) Gold sol is lyophilic sol, gelatin acts as stabilising agent
(c) Gelatin produces negative charge on gold particles in gold sol
(d) Gelatin helps gold sol to get its critical micelle concentration
Answer:
(a) Gold sol is lyophobic sol, gelatin acts as stabilising agent
Question 43.
Which of the following is not a method for coagulation of lyophobic sols ?
(a) By electrophoresis
(b) By mixing oppositely charged sols
(c) By adding electrolyte
(d) By adding a protective colloid
Answer:
(d) By adding a protective colloid
Question 44.
What happens when a lyophilic sol is added to a lyophobic sol ?
(a) Lyophilic sol is protected
(b) Lyophobic sol is protected
(c) Both the sols are coagulated
(d) Electrophorsis takes place
Answer:
(b) Lyophobic sol is protected
![]()
Question 45.
Which of the following is not a correct match ?
(a) Butter- OW type emulsion
(b) Vanishing cream-O/W type emulsion
(c) Milk – OW type emulsion
(d) Cream – W/O type emulsion
Answer:
(a) Butter- OW type emulsion
Question 46.
Which of the following is not an example of an emulsifying agent ?
(a) Proteins
(b)Gums
(c) Soaps
(d) Electrolytes
Answer:
(d) Electrolytes
Question 47.
The separation of an emulsion into its constituent liquids is known as
(a) emulsification
(b) protection of colloid
(c) coagulation
(d) demulsification
Answer:
(d) demulsification
Question 48.
Which of the following processes does not occur at the interface of phases ?
(a) Crystallisation
(b) Heterogeneous catalysis
(c) Homogeneous catalysis
(d) Corrosion
Answer:
(c) Homogeneous catalysis
Question 49.
At the equilibrium position in the process of adsorption …………
(a) ΔH > 0
(b) ΔH = TΔS
(c) ΔH>TΔS
(d) ΔH<TΔS
Answer:
(b) ΔH = TΔS
![]()
Question 50.
Which of the following interfaces cannot be obtained ?
(a) Liquid-Liquid
(b) Solid-Liquid
(c) Liquid-Gas
(d) Gas-Gas
Answer:
(d) Gas-Gas
Question 51.
The term ‘sorption’ stands for………………
(a) absorption
(b) adsorption
(c) both absorption and adsorption
(d) desorption
Answer:
(c) both absorption and adsorption
Question 52.
Extent of physisorption of a gas increases with……………
(a) increase in temperature
(b) decrease in temperature
(c) decrease in surface area of adsorbent
(d) decrease in strength of van der Waals forces
Answer:
(b) decrease in temperature
Question 53.
Which one of the following is not applicable to the phenomenon of adsorption ?
(a) ΔH > 0
(b) ΔG < 0
(c) ΔS < 0
(d) ΔH < 0
Answer:
(a) ΔH > 0
Question 54.
Which of the following is not a favourable condition for physical adsorption ?
(a) High pressure
(b) Negative ΔH
(c) Higher critical temperature of adsorbate
(d) High temperature
Answer:
(d) High temperature
Question 55.
Physical adsorption of a gaseous species may change to chemical adsorption with……………
(a) decrease in temperature
(b) increase in temperature
(c) increase in surface area of adsorbent
(d) decrease in surface area of adsorbent
Answer:
(b) increase in temperature
![]()
Question 56.
Which of the following is an example of absorption ?
(a) Water on silica gel
(b) Water on calcium chloride
(c) Hydrogen on finely divided nickel
(d) Oxygen on metal surface
Answer:
(b) Water on calcium chloride
Question 57.
At high concentration of soap in water, soap behaves as ……..
(a) molecular colloid
(b) associated colloid
(c) macromolecular colloid
(d) lyophilic colloid
Answer:
(b) associated colloid
Question 58.
Method by which lyophobic sol can be protected ……….
(a) by addition of oppositely charged sol
(b) by addition of an electrolyte
(c) by addition of lyophilic sol
(d) by boiling
Answer:
(c) by addition of lyophilic sol
Question 59.
Freshly preparad precipitate sometimes gets converted to colloidal solution by……………
(a) coagulation
(b) electrolysis
(c) diffusion
(d) peptisation
Answer:
(d) peptisation
![]()
Question 60.
Which of the following electrolytes will have maximum coagulating value for Agl/Ag+ sol ?
(a) Na2S
(b) Na3PO4
(c) Na2SO4
(d) NaCI
Answer:
(b) Na3PO4
Question 61.
Which of the following process is responsible for the formation of delta at a place where rivers meet the sea ?
(a) Emulsification
(b) Colloid formation
(c) Coagulation
(d) Peptisation
Answer:
(c) Coagulation
Question 62.
Which of the following process is not responsible for the presence of electric charge on the sol particles ?
(a) Electron capture by sol particles
(b) Adsorption of ionic species from solution
(c) Formation of Helmholtz electrical double layer
(d) Adsorption of ionic species from solution
Answer:
(d) Adsorption of ionic species from solution