BSEB Bihar Board 12th Chemistry Important Questions Short Answer Type Part 1 are the best resource for students which helps in revision.
Bihar Board 12th Chemistry Important Questions Short Answer Type Part 1 in English
Question 1.
Write chief ore of Zinc.
Answer:
Following chief ores of Zinc ore-
(a) Calamine – ZnCO3
(b) Zinc Blende – ZnS.
(c) Zincite – ZnO
(d) Frantelinite – ZnO·Fe2O3
Question 2.
What is Gangue.
Answer:
The Impurities present in the Phase of ores of metal known as Gangue.
Example. Silica (SiO2) CeO, CaO etc.
Question 3.
What is Hybridisation of Carbon in CH4.
Answer:
Sp3.
Question 4.
What is the formula of oleum.
Answer:
H2S2O7
Question 5.
What is Nitroleum.
Answer:
Mixture of carbon-and Calcium cyanamide known as Nitorleum i.e. CaNCN + C
Question 6.
What is the oxidation Number of Phosphorous in H3PO3.
Answer:
+3
Question 7.
What is Geometrical shape of sp3.
Answer:
Tetrahedral.
Question 8.
What is Geometrical shape of d2sp3.
Answer:
Octahedral.
Question 9.
Give examples of Amorphous Solid.
Answer:
Glass, Rubber, Plastics, Protein, Starch.
Question 10.
What is Geometrical shape of NH3.
Answer:
Pyramidal.
Question 11.
What is structure of Ozone molecule.
Answer:
Triangular
Question 12.
What is formula of Caro’s acid.
Answer:
H2SO5
Question 13.
What is formula of Mashall acid.
Answer:
H2S2O8
Question 14.
What is coordination Number of each ions in Rocksalt costal.
Answer:
6
Question 15.
Which Point defect charges density of solid in crystal unit cell.
Answer:
Schottky defees.
Question 16.
The solid in which Both Frenkel and schottky defect founds.
Answer:
AgBr.
Question 17.
What is Packing efficiency in follow in unit cell.
(a) Simple cubic unit cell
(b) Body-centred cubic unit cell
(c) Hexagonal close Packed Lattices
Answer:
The Packing efficiency of different unit cell are Giyen below-
(a) Simple cubic = 52.4%
(b) Body centred cubic = 68%
(c) Hexogonal close Packed = 74%
Question 18.
What types of stoichiometric defect are shown by ZnS.
Answer:
ZnS crystal shows Frenkel defects. In ZnS. Size of Cation is smaller as compared to the size of Anion.
Question 19.
Give examples of Anyjrphours solids.
Answer:
Glass, Rubber, Plastics, Strach, Protein etc.
Question 20.
Give examples of n-type extrinsic semiconductors.
Answer:
(a) Silicon containing P or As as Impurites.
(b) Germanium Containing P or As as the Impurities.
Question 21.
Give examples of P-types extrinsic semiconductors.
Answer:
(a) Silicon (Si) Containing B, or AI as Impurity.
(b) Germanium (Ge) Containing B or Al as Impurity.
Question 22.
How many atoms are present in Body centred cubic unit.
Answer:
2.
Question 23.
How many atoms are present in Face centred cubic unit cell.
Answer:
4.
Question 24.
What is radius ratio.
Answer:
The ratio of the radius of cation and radius of anion is called radius ratio.
Radius ratio = \(\frac{\text { Radius of cotton(r } \left.^{+}\right)}{\text {Radius of Anion }\left(r^{-}\right)}\)
Question 25.
What is Dry ice.
Answer:
Solid CO2 is called as Dry ice.
Question 26.
What is Coordination Number of Body centered cubic unit cell.
Answer:
8.
Question 27.
What is coordination Number of h.c.p or c.c.p crystal.
Answer:
12.
Question 28.
What is the effect of Temperature on conductivity of semiconductor.
Answer:
Conductivity of Semiconductor is increases with rise in Temperature.
Question 29.
Give example of Antiferromagnetic.
Answer:
Cr2O3, Fe2O3, MnO, NiO·MnO2 etc.
Question 30.
How many edges are present in a cube.
Answer:
6.
Question 31.
What is molarity and molality of Pure water at 21°C.
Answer:
Both Molarity and molality of Pure water at 25°C is same which equal to 55.5.
Question 32.
What is vant Hoff factor.
Answer:
Vant Hoff factors is denoted by ‘i’ Ratio of observed colligative Property and Normal colligative property of the solution is known as Vant Hoff factor.
Vant Hoff Factor (i) = \(\frac{\text { Observed colligative Property }}{\text { Normal colligative Property. }}\)
Question 33.
What is azeotropic Mixture.
Answer:
The Mixture which boils at constant temperature with change their composition known as Azeotropic Mixutre or Azeotrops.
Question 34.
What is Ideal solution.
Answer:
The solution which follows Raoult’s Law at all temp and concentration.
i.e. (i) PA = XA · PA0, PB = XB · PB0
(ii) ΔHMixing = 0
(iii) ΔVMixing = 0
Question 35.
What is Non-Ideal solution.
Answer:
A Solution Which is not follow to Raoult’s Law, Known as Non-Ideal solution.
(i) PA ≠ XA · PA0, PB ≠ XB · PB0
(ii) ΔHMixing ≠ 0
(iii) ΔVMixing ≠ 0
Question 36.
What is Isotonic solutions.
Answer:
Those solution which have same osmotic Pressure, is called isotonic solution.
Question 37.
Write Name of d-BIock elements.
Answer:
Transition Metal.
Question 38.
Write Name of f-Block Elements.
Answer:
Inner Transition Metal.
Question 39.
Write Formula of Green Vitriol.
Answer:
FeSO4·7H2O.
Question 40.
Write Name of metals present in Brass.
Answer:
Zn and Cu.
Question 41.
Write Name of Meta! Present in Vitamine B-12.
Answer:
Cobalt.
Question 42.
A 5 % solution of Cane-Sugar (molecular Mass = 342) is Isotonic with 1% solution of Substance X. The Molecule weight of X is-
Answer:
68.4
Question 43.
Write IUPAC Name of following.
Answer:
(a) [CO(NH3)4] Cl2 = Tetra ammine cobalt (H) chloride .
(b) K4 [Fe(CN)6] = Potassium hexa Gyano Ferrate (II)
(c) K2 [Ni(CN)4] = Potassium tetra cyano Nickelate (II)
(d) [COCl2 (NH3)4]Cl = Teta ammine dichloro cobalt (III) chloride
(e) [PtCl4]-2 = Tera chloro Platinate (II)
Question 44.
Write the Structure formula of the following-
(a) 4, 4 – Dimethyl – 2 – Pentanol (b) 2 – Butanol
Answer:

Question 45.
Write the structure of
2-Chloro-3-Methyl Pentane.
Answer:
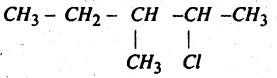
Question 46.
Write the structure of
1-Bromo, -4-Sec-Butyl- 2- Methyl Benzene.
Answer:
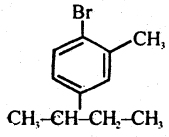
Question 47.
Write IUPAC Name of the Compound
Answer:
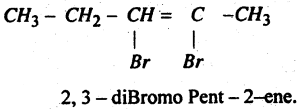
Question 48.
How does a catalyst effect the rate of a reaction.
Answer:
A catalyst may Increases or decrease the rate of reaction.
Question 49.
Define the term Tyndall Effect.
Answer:
It is defined as the scattering of light by the colloidal particles present in a colloidal solution.
Question 50.
What is CMC (Critical Micelle Concentration).
Answer:
The formation of micelles take place only above certain concentration is called critical micelle concentration (C.M.C.)
Question 51.
What is Kraft Temperature.
Answer:
The formation of micelles take place only above a certain Temperature, called Kraft Temperature.
Question 52.
What is Slag.
Answer:
A mixture of flux and gangue is called slag.
Gangue + Flux → Slag.
Question 53.
Arrange the following sets of compounds in order of their Increasing Boiling point.
Pentan -1-01, Butan-1-01, Butan-2-01, Ethanol, Propan-1-01, Methanol.
Answer:
Methanol < Ethanol < Propan-1-01 < Butan-1-01 < Pentan-1-01.
Question 54.
(a) Complete the following reaction-
![]()
Answer:
![]()
Question 54.
(b) Complete the following reaction-
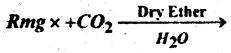
Answer:
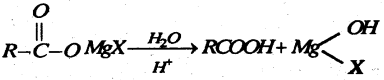
Question 55.
What are Lanthanides.
Answer:
First Inner Transition series (4f-series) called Lanthanides.
Question 56.
Give the main reaction in Alumino thermic process.
Answer:
Cr2O3 + 2Al → 2Cr + Al2O3
Question 57.
What are Actinides.
Answer:
Second Inner Transition series (5f-series) Called Actinides.
Question 58.
(a) Arrange the following in increasing order of their and strength-Propan-1-01, 2, 4, 6 Prinitro-Phenol, 3-nitro phenol, 3,5- dinitrophenol, Pheno, 4-methyl Phenol.
Answer:
Propan-1-01 < 4-Methyl Phenol < Phenol < 2,4,6-trinitro phenol. < 3,5,= dinitro pheno, < 3-nitro phenol.
Question 58.
(b) Arragne in the Increasing order of Aud strength.
CCl3COOH, CH3COOH, CHCl2COOH, CH2Cl COOH.
Answer:
CH3COOH < CH2Cl COOH < CHCl2 COOH <CCl3COOH.
Question 59.
(a) Name the product.
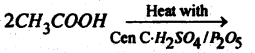
Answer:
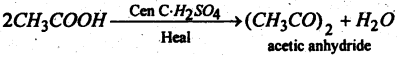
Question 59.
(b) Complete the reaction-
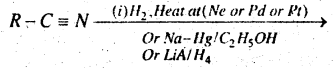
Answer:
RCH2NH2
Question 60.
(a) What is the product in the reaction.

Answer:
RCH2 NH2 + H2O
Question 60.
(b) Complete the reaction-
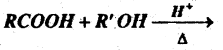
Answer:
RCOOR’+ H2O