BSEB Bihar Board 12th Chemistry Important Questions Short Answer Type Part 3 are the best resource for students which helps in revision.
Bihar Board 12th Chemistry Important Questions Short Answer Type Part 3 in English
Question 1.
Aldehydes are more reactive than ketones. Explain.
Answer:
Due to smaller +I effect of the alkyl group in a aldehydes as compared to large +I effect of two alkyl groups in ketones, the magnitude of positive charge on the carbonyl carbon is more in aldehydes that ketones. As a result nucleophilic addition reaction occur more readily in aldehydes that in ketones.
As the number and size of the alkyl groups around C = O group increases, there is steric hindrance which makes the attack of the nucleophile on the carbonyl carbon more difficult.
Question 2.
Explain the terms polymer and monomer.
Answer:
Polymer- Polymer is a high molecular mass macromolecules consisting of repeating structural units derived from monomers. Polythene, bakelite, polyvinyl chloride, nylon 6 etc. are polymers.
Monomer- Monomer is a simple molecule capable of undergoing polymerisation and leading to the formation of the corresponding polymer. For examples ethane, vinyl chloride etc.
Question 3.
How do the antimicrobials control the microbial disease?
Answer:
Antimicrobials control the microbial disease in three ways :
- Bactericidal drugs kill the organisms in the body.
- Bacteriostatic drugs inhibit or arrest the growth of organisms.
- Antimicrobials increase the immunity and resistance of infection of the body.
Question 4.
Classify the polymers on the basis of structures.
Answer:
On the basis of structured polymers are classified as follows :
- Linear polymers e.g polyvinyl chloride, polythene etc.
- Branched chain polymers eg. low density polythene.
- Cross-liked polymers eg. bakelite, melanine etc.
Question 5.
Name an alkaloid in each case used for the treatment of: (a) hypertension, (b) malaria, (c) pain.
Answer:
- Hypertension- Reserpine is the alkaloid used in the treatment of hypertension.
- Malaria- Quinine, an alkoloid used in the treatment of malaria.
- Pain- Morphene, an alkaloid used in the. treatment of pain.
Question 6.
What happens when :
(i) Ethanol is heated with cone, sulphuric acid at 100° C
(ii) Formaldehyde is warmed with caustic potash solution.
(iii) Acetaldehyde is treated with aqueous caustic soda solution.
(iv) Acetone is heated with iodine and caustic soda solution.
Answer:
(i) Ethanol when heated with cone, sulphuric acid at 100°C, ethyl hydrogen sulphate is obtained.
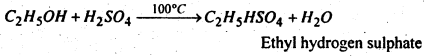
(ii) Formaldehyde when heated with caustic potash solution, methyl alcohol and potassium formate are formed.
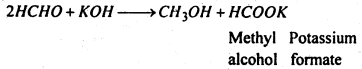
(iii) When acetaldehyde is treated with NaOH solution, aldol is obtained.
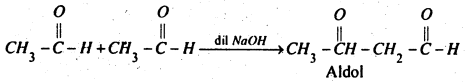
(iv) Acetone when heated with iodine and caustic soda solution, iodoform is formed.
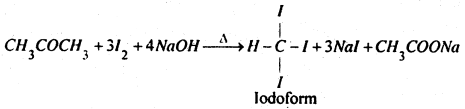
Question 7.
Complete and balance the following equations-
Answer:
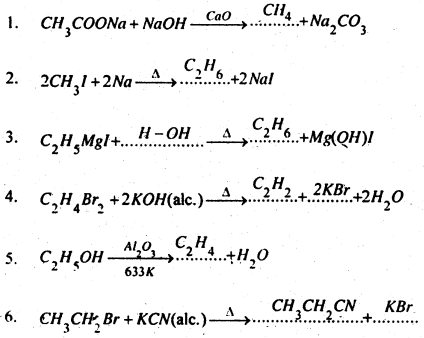


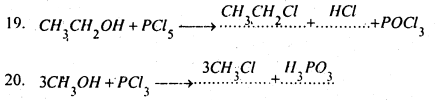
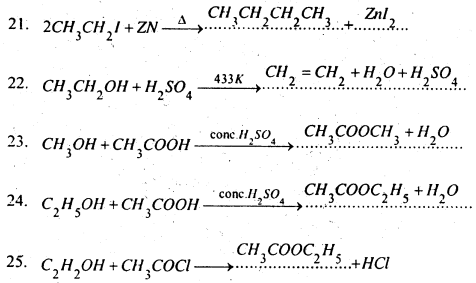

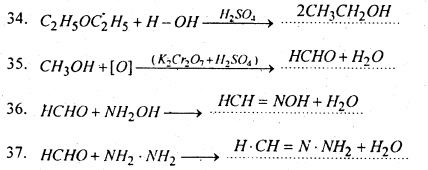


Question 8.
Identify the products-
![]()
Answer:
(A) CH3COONa; (b) CH4; (C) CH3Cl
![]()
Answer:
(A) C2H5Br ; (B) C2H5CN ; (C) C2H5COOH
![]()
Answer:
(A) C2H6 ; (B) C2H5Cl; (C) C2H5OH
![]()
Answer:
(A) CH3OH; (B) HCHO
![]()
Answer:
(A) CH3CHO: (B) CH3·CH = N·NH2
6. \(\mathrm{CH}_{3} \mathrm{CHO} \stackrel{\mathrm{HCN}}{\longrightarrow}(\mathrm{A}) \stackrel{\mathrm{H}_{2} \mathrm{O}, \mathrm{H}^{+}}{\longrightarrow}(\mathrm{B})\)
Answer:
(A) CH3CH(OH)CN; (B) CH3CH (OH)·COOH (lactic acid)
![]()
Answer:
(A) CH3CHO; (B) CH3CH = NOH
![]()
Answer:
(A) CH3Cl; (B) C2H6
![]()
Answer:
CH3CHO; (B) CH3COOH ; (C) (CH3COO)2 Ca
![]()
Answer:
(A) CH3Br, (B) CH3MgBr
![]()
Answer:
(A) CH3CH2OH ; (B) CH3CHO
![]()
Answer:
(A) CH3COONH4 ; (B) CH3CONH2 ; (C) CH3NH2
Question 9.
Give reasons for the following observations :
(i) SF6 is not easily hydrolysed though thermodynamically it should be.
Or, SF6 is inert towards hydrolysis.
(ii) Sulphur exhibits greater tendency for catenation than selenium.
Answer:
(i) in SF6, S is sterically protected by six F atoms and hence does not allow H2O molecules to attack the S atom. Further, F does not have d-orbitals to accept the electrons donated by H2O molecules. As a result of these two reasons, SF6 does not undergo hydrolysis. On the other hand, in SF4, S is not sterically protected since it Is surrounded by only four F atoms. Thus, attack of H2O molecules can take place easily and hence hydrolysis occurs.
(ii) As we move from S to Se, the atomic size increases and hence the strength of E-E bond decreases. Thus, S-S bond is much stronger than Se-Se bond consequently, S shows greater tendency for catenation than selenium.
Question 10.
Account for the following :
(i) Bond dissociation energy of F2 is less than that of Cl2.
(ii) Both NO and ClO2 are odd electron species but NO dimerises while ClO2 does not.
(iii) Bleaching of flowers by chlorine is permanent while that by sulphur dioxide is temporary.
Answer:
(i) Fluorine atom being smaller in size, the interelectronic repulsions between the non-bonding electrons, present in the 2p-orbitals of fluorine atoms are much larger than similar interelectronic repulsion in the 3p-orbitals of chlorine atoms.
(ii) In NO, the odd electron on N is attracted by only one 0-atom but in ClO2, the odd electron on Cl is attracted by two O-atoms. Thus, the odd electron on N in NO is localised while the odd electron on Cl in ClO2 is delocalised. Consequently, NO has a tendency to dimerise but ClO2 does not.
(iii) Cl2 bleaches coloured material by oxidation :
Cl2 + H2O → 2 HCl + O
Coloured material + [O] → Colourless
and hence bleaching is permanent.
In contrast, SO2 bleaches coloured material by reduction and hence bleaching is temporary since when the bleached colourless material is exposed to air, it gets oxidised and the colour is restored.
SO2 + 2H2O → H2SO4 + 2H

Question 11.
Assign appropriate reasons for each of the following statements:
Metal fluorides are more ionic in nature than metal chlorides.
(ii) Hydrogen fluoride is a weaker acid than hydrogen chloride in aqueous solution.
(iii) Addition of Cl, to KI solution gives it a brown colour but excess of Cl2 turns it colourless.
(iv) Perchloric acid is a stronger add than sulphuric acid.
Answer:
(i) According to Fajan rules, a bigger anion is more easily polarised than a smaller anion. As a result, same metal cation can polarise a bigger Cl– ion more easily than the smaller F– ion. In other words, for the same metal, the metal fluoride is more ionic than metal chloride. So,. in general, we can easily say that metal fluorides are ionic than metal chlorides.
(ii) Due to the smaller size of F as compared to Cl, the bond dissociation energy of H-F bond is much higher than that.of H-Cl bond.Thus, in aqueous solution, H-Cl bond can break more easily to form H+ ion than H-Cl bond. Hence, hydrogen fluoride is a weaker acid than hydrogen chloride in aqueous solution.
(iii) Cl2 being a stronger oxidising agent than I2, first oxides KI to give I2 which imparts brown colour to the solution.
![]()
If Cl2 is passed in excess, the I2 thus formed gets further oxidised to iodic acid (HIO3) which is colourless.
![]()
(iv) The oxidation state of Cl in perchloric acid is +7 while that of S in sulphuric acid is +6.
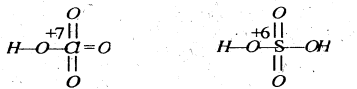
Thus, ClO3 part of HClO4 can break the O-H bond more easily to liberate a proton than the SO2 part in H2SO4. Therefore, perchloric acid is a stronger acid than sulphuric acid.
Question 12.
(1) Why HF add is stored in wax coated glass bottles
(2) CIF3 exists but FCl3 does not.
Answer:
(1) HF does not attack wax but reacts with glass. It dissolves SiO2 present in glass forming hydrofluorosilicic acid.
SiO2 + 6HF → H2SiF6 + 2H2O
(2) It is due to the following reason :
(i) Cl has vacant d-orbitals and hence can show an oxidation state of +3 but F has no d-orbitals, so, it cannot show positive oxidation states. Since F can show only -1 oxidation state, FCl3 does not exist.
(ii) Because of bigger size, Cl can accommodate three small Fatoms around it while F being smaller cannot accommodate three large sized Cl atoms around it.
Question 13.
Why arc halogens coloured?
Answer:
All halogens are coloured. It is due to the reason that their molecules absorb light in the visible region as a result of which their electrons get excited to higher energy levels while the remaining light is transmitted. The colour of the halogens is actually the colour of this transmitted light. The amount of energy needed for excitation decreases progressively from F to I as the size of the atom increases. Hence, the energy of the transmitted light goes on increasing from F to I.
In other words, the colour of the halogens deepens from F2 to I2. For example, F2 absorbs violet light and hence appears pale yellow while iodine absorbs yellow and green light and hence appears deep violet. In the same way, we can account for greenish yellow colour of Cl2 and orange red colour of bromine.
Question 14.
Write the reactions of F2 and Cl2 with water.
Answer:
F2 being a strong oxidising agent oxidises H2O or O3.
![]()
Cl2 reacts with H2O to form hydrochloric acid and hypochlorous acid.

Question 15.
How can you prepare Cl2 from HCl and HCl from Cl2 ? Write reactions only.
Answer:
HCl can be oxidised to Cl2 by a number of oxidising agents like MnO2, KMnO4 and K2CrO7.
MnO2 + 4HCl → MnCl2 + Cl2 + 2H2O
Cl2 can be reduced to HCl by reaction of H2 in the presence of diffused sunlight.
![]()
Question 16.
How are XeF2, XeF4 and XeF6 obtained ?
Answer:
These xenon fluorides are prepared by direct reaction between Xe and F2 under differentiation as shown below:
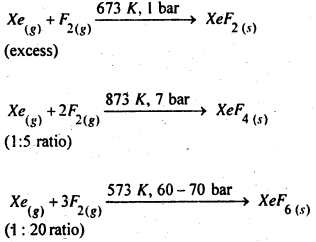
Question 17.
How are XeO3 and XeOF4 prepared?
Answer:
Hydrolysis of XeF4 and XeF6 with water gives XeO3.
6 XeF4 + 12 H2O → 4Xe + 2 XeO3 + 24 HF + 3 O2
XeF6 + 3H2O → XeO3 + 6HF
In contrast, partial hydrolysis of XeF6 gives XeOF4
XeF6 + H2O → XeOF4 + 2HF
Question 18.
(1) Complete the following reaction equations :
(1) Ca (OCl)2 + HCl →
(ii) NaClO3 (aq) + SO2 (g) →
(2) F-atom is more electronegative than I-atom, yet HF has lower add strength than HI. Explain.
Answer:
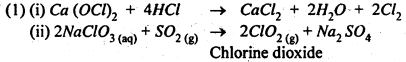
(2) F-atom is smaller in size than I-atom. Hence the bond dissociation energy of H-F is high as compared to that of H-I bond. Therefore, H-I bond breaks more easily than H-F bond and thus HI is a stronger acid than HF.
Question 19.
Given reasons :
(i) Phosphine is a weaker base than NH3.
Or, NH3 has a higher proton affinity than PH3.
(ii) NO (Nitric oxide) is paramagnetic in the gaseous state but diamagnetic in the liquid and solid states.
Answer:
(i) When NH3 or PH3 accepts a proton, an additional N-H or, P-H bond is formed.
H3N: + H+ → NH4+; H3P: + H+ → PH4+
Due to the bigger size of P than N, P-H bond thus formed is much weaker than the N-H bond. Thus, PH3 has less tendency than NH3 to accept a proton. Hence, PH3 is weaker base than NH3 or NH3 has a higher proton affinity than PH3.
(ii) NO has an odd number of electrons (11 valence electrons) and hence is paramagnetic in the gaseous state. But in liquid and solid states, it exists as a symmetrical or asymmetrical dimer and hence is diamagnetic in these states.
Question 20.
Give reasons for the following :
(i) Noble gases are mostly inert.
(ii) Why do noble gases form compounds with fluorine and oxygen only?
(iii) Neon is generally used for warning signals.
Answer:
(i) Noble gases are mostly inert because of the following reasons :
- They have completely filled ns2 np6 electronic configurations in their valence shells.
- Electron gain enthalpies of noble gases are positive.
- They have high ionization enthalpies.
(ii) Fluorine and oxygen are the most electronegative elements and hence are very reactive, they form compounds with noble gases, particularly xenon.
(iii) Neon lights are visible from long distances even in fog and mist and hence neon is gas used for warning signals.